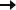STUDY DESIGN
Caregivers of patients living with LGS were surveyed to assess patients’ overall condition while taking EPIDIOLEX
In developmental and epileptic encephalopathies like LGS, epileptic activity may contribute to severe cognitive and behavioral impairments beyond what might be expected from the underlying pathology alone.3
Subject/Caregiver Global Impression of Change (S/CGIC) survey: A key secondary endpoint
In LGS Study 1 and Study 2, changes from baseline score in the S/CGIC survey at the last visit were analyzed. To determine S/CGIC score, caregivers referenced written descriptions of patients’ overall condition at study initiation as a memory aid and rated the following question on a 7-point scale:
“Since [you/your child] started treatment, please assess the status of [your/your child’s] overall condition (comparing [your/their] condition now to [your/their] condition before treatment) using the scale below”

S/CGIC
Improvements in overall condition with EPIDIOLEX were reported by more caregivers in the LGS pivotal trials4,5
A greater improvement in S/CGIC score was reported in patients treated with EPIDIOLEX compared with placebo in LGS Study 1 and Study 2.4,5
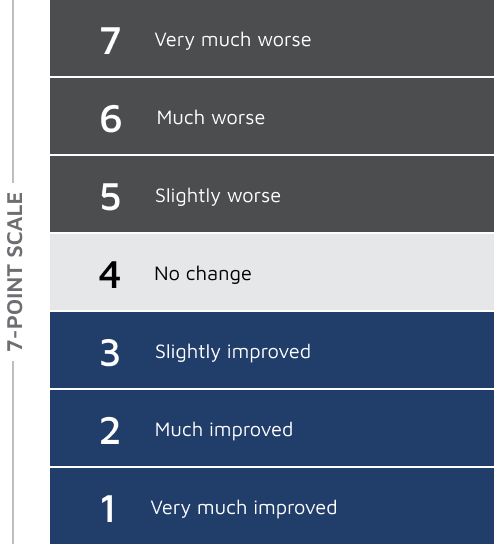
S/CGIC SURVEY: Change from baseline S/CGIC score4,5

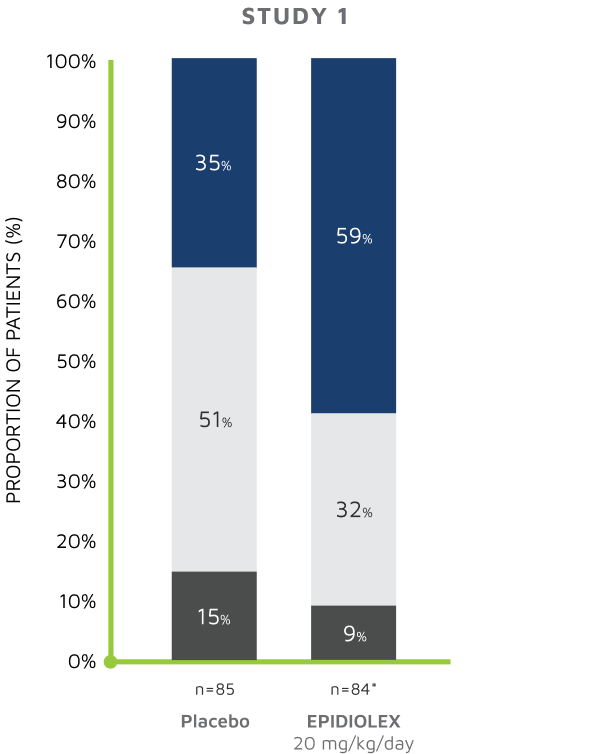
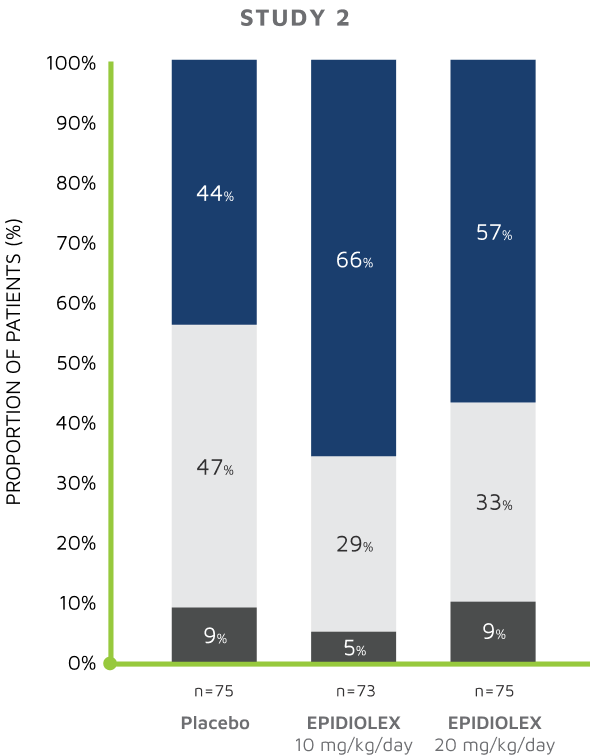
In Study 1, the mean S/CGIC score at last visit was 3.0 (“slightly improved”) for EPIDIOLEX 20 mg/kg/day compared with 3.7 (“no change”) for placebo (P<0.01). In Study 2, the mean S/CGIC score at last visit was 3.0 and 3.2 (“slightly improved”) for EPIDIOLEX 10 mg/kg/day and 20 mg/kg/day, respectively, compared with 3.6 (“no change”) for placebo (P<0.01 and P=0.04, respectively).
*The questionnaire was not completed for 2 patients in the cannabidiol group.4
LEARN MORE
THE BECOME SURVEY
The BECOME (behavior, cognition, and more with EPIDIOLEX) survey
The BECOME survey sought to further clarify the results of the S/CGIC ratings from the LGS pivotal trials by investigating the impact on seizure domains and other condition-related domains, more specifically through the collection of real-world feedback.1,2,6
At baseline, 55% of patients in the BECOME survey were male, median age was 17 years, the median number of current ASMs was 4, and the median dose of EPIDIOLEX was 14 mg/kg/day.1,2
Real-world data
The BECOME survey captured caregiver impressions of patients living with LGS in seizure domains and other condition-related domains.1,2
Outcomes compared to before starting treatment
In an online survey of 396 US-based caregivers of patients living with LGS taking EPIDIOLEX ≥3 months, respondents were asked to compare the past month to the period prior to initiation of CBD.1,2,6*†
Open label, non-placebo controlled exploratory study
Results from this exploratory study are descriptive only and the limitations must be considered. The BECOME survey was an open-label, non-placebo controlled study. Retrospective studies may have the potential for “recall bias” due to the lack of the respondent’s documented baseline.1,2
Domains assessed1,2
- Seizure frequency
- Seizure-free days
- Seizure severity
- Alertness, cognition, and executive function
- Emotional and social functioning
- Language and communication
*Patients were excluded if using medical marijuana or other non–FDA approved CBD or cannabinoid product. “Don’t recall” or “Not applicable” responses were excluded.1,2
†The survey consisted of multiple choice and rank order questions based on questions from validated measures and other previously published caregiver reports, using symmetrical 3-, 5-, and 7-point Likert scale, depending on the domain (from worsening to improvement).1,2
Results of the BECOME survey
Caregiver impressions of patients living with LGS taking EPIDIOLEX7
For all domains in the BECOME survey, caregivers were asked to compare the past month to the period prior to initiation of CBD.1,2
Limitations include retrospective caregiver accounts and selection bias due to study design.1,2
"Any worsening" and "any improvement" includes definite major, definite minor, and possible changes.
ISOLATE DATA:
Select up to 3
Caregiver-reported change7
Seizure frequency
Any worsening
No clear change
Any improvement
Any worsening
No clear change
Any improvement
Seizure frequency
Any increase
No clear change
Any decrease
Any increase
No clear change
Any decrease
Seizure-free days
Fewer seizure-free days per week
No clear change
More seizure-free days per week
Seizure severity
Any worsening
No clear change
Any improvement
Seizure severity
Any increase
No clear change
Any decrease
Caregiver-reported change in ability to7:
Alertness, cognition, and executive function
Any worsening
No clear change
Any improvement
Caregiver-reported change in how often they7:
Emotional and social functioning
Any worsening
No clear change
Any improvement
Caregiver-reported change in ability to7:
Nonverbal language and communication
Any worsening
No clear change
Any improvement
Verbal language and communication
Any worsening
No clear change
Any improvement
LEARN MORE
Similar resources

Dravet syndrome data
EPIDIOLEX significantly reduced convulsive seizures in patients living with Dravet syndrome
See the data

TSC data
EPIDIOLEX reduced the frequency of TSC-associated seizures
See the data

Safety
The safety profile of EPIDIOLEX was evaluated in an expansive clinical trial program
See the data